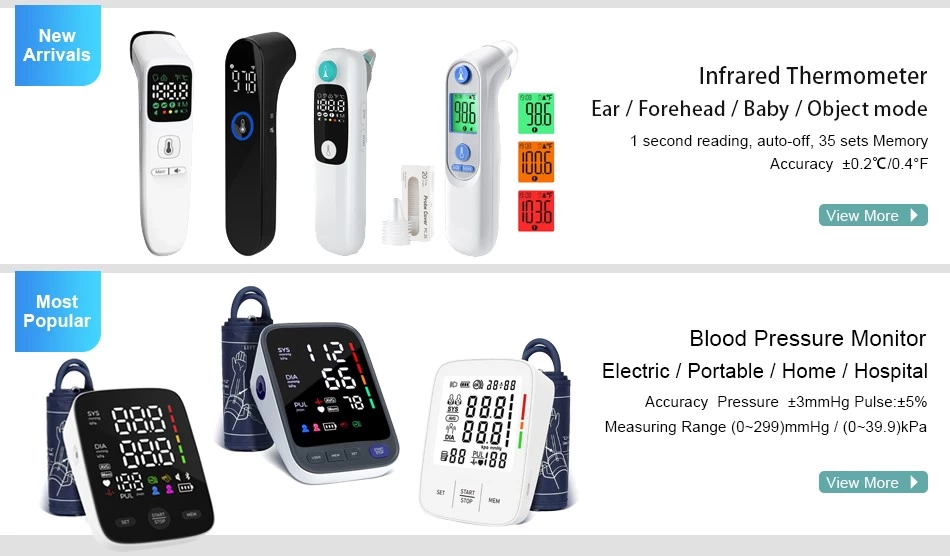
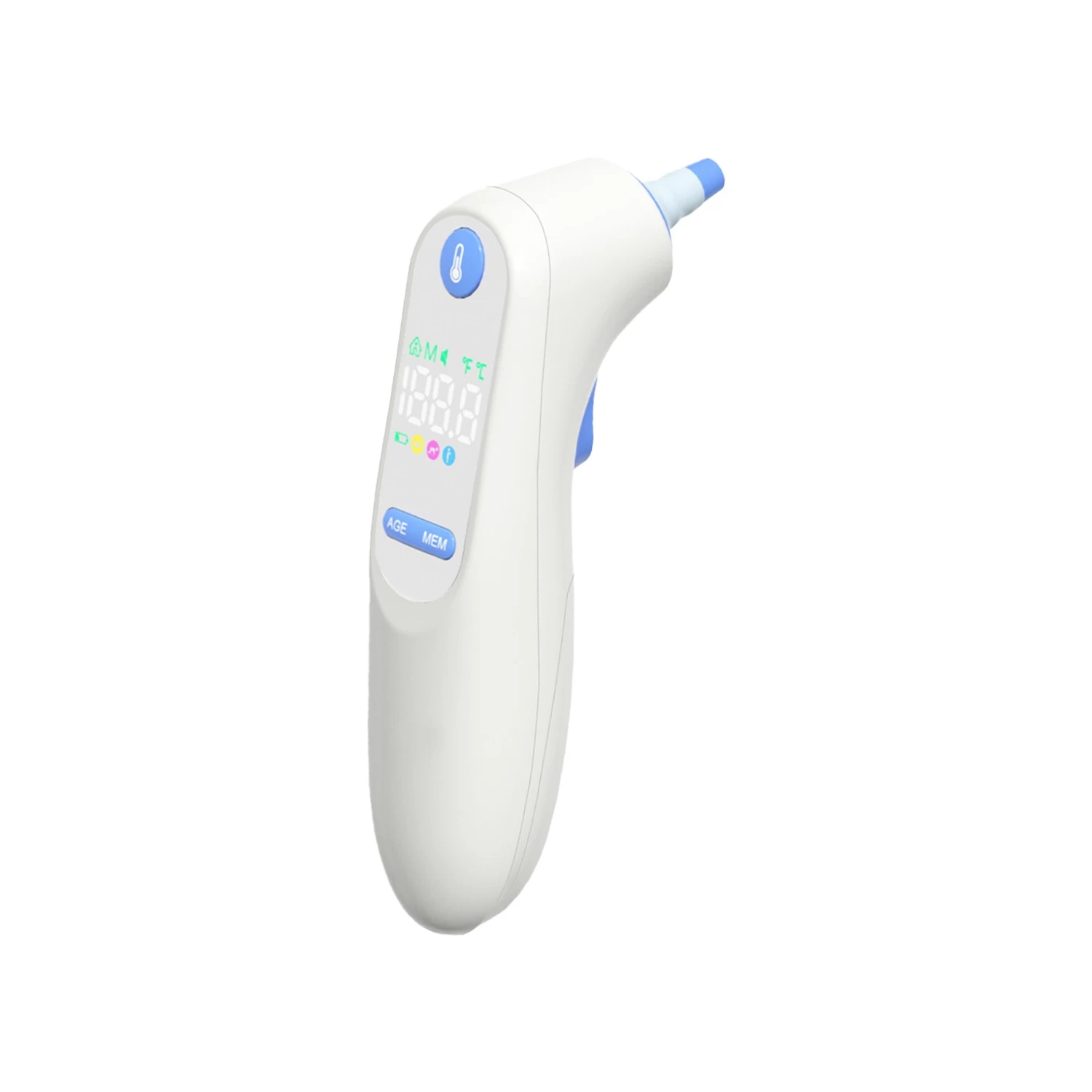
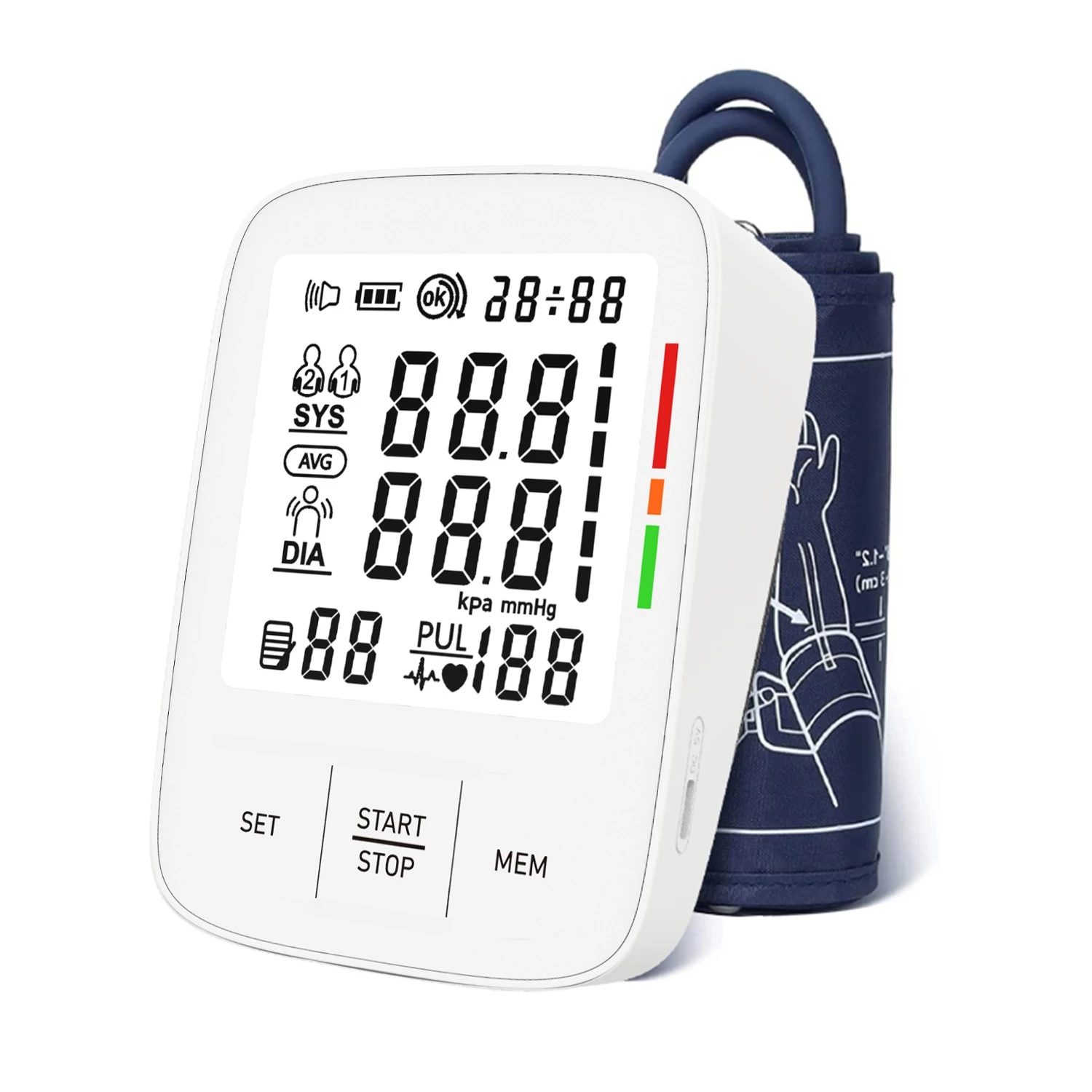
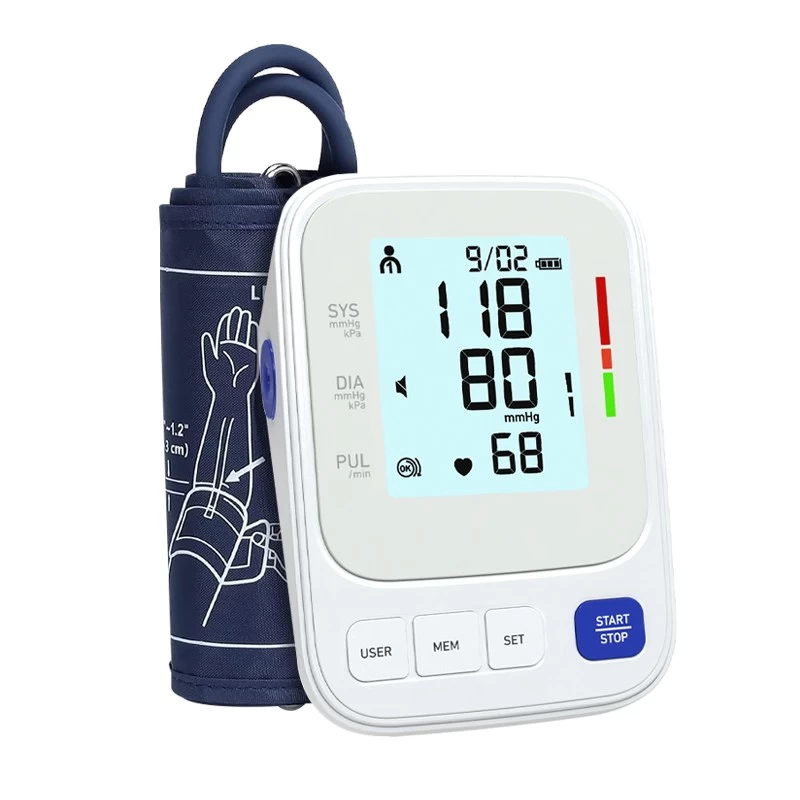
Fincare Achieves New Milestone in Medical Device Compliance: 47 Product Models Secure EU MDR Certification
Fincare Achieves New Milestone in Medical Device Compliance: 47 Product Models Secure EU MDR Certification
Fincare has recently reached a significant milestone in medical device compliance—47 of its product models have successfully obtained certification under the EU Medical Device Regulation (MDR). This achievement underscores Fincare’s strong commitment to product quality, safety, and regulatory compliance, while further strengthening its leadership in the global medical device market.

I. EU MDR Certification: The Global “Gold Standard” for Medical Devices
Since officially replacing the previous Medical Device Directive (MDD) in 2021, the EU MDR has become one of the most stringent and authoritative regulatory frameworks for medical devices worldwide. The regulation aims to significantly enhance patient safety and strengthen oversight throughout the entire lifecycle of medical devices. Key aspects include:
-
Stricter Clinical Data Requirements: Mandating comprehensive, accurate, and reliable clinical evaluation reports.
-
Enhanced Risk Management: Covering all stages from product design and manufacturing to usage and disposal.
-
Improved Traceability: Ensuring each product can be traced back to its origin.
-
Robust Post-Market Surveillance: Emphasizing continuous monitoring and timely response to market feedback.
Securing MDR certification for all 47 product models—spanning a range of clinical and home-use devices—reflects Fincare’s unwavering dedication to quality, agility in adapting to regulatory updates, and strategic foresight in global market positioning.
II. Strengthening Presence in Europe and Advancing Global Strategy
This certification opens significant opportunities for Fincare to expand its business across the European market. With MDR certification, more Fincare products will gain smoother access to EU medical systems, including public hospitals, private clinics, and pharmacy networks. It also paves the way for partnerships with high-quality distributors and hospital groups. This achievement is not only a compliance victory but also a strategic step forward.
Fincare remains committed to delivering safer, more convenient, and efficient medical solutions to patients worldwide.
Driving Innovation and Building Future-Ready Compliance Capabilities
Amid increasingly strict global regulations, Fincare goes beyond merely “meeting standards.” Through a dual-driven approach of “compliance and innovation,” the company continues to invest in:
-
Product R&D: Strengthening core technological capabilities and enhancing real-world user experience and efficacy.
-
Systematic Regulatory Response: Maintaining a global regulatory database and expert team to dynamically adapt to regional requirements.
-
Partnership Ecosystem: Elevating collaboration with global partners through higher standards and shared values.
III. Looking Ahead: Delivering Trusted Medical Products Worldwide
The certification of 47 product models under the EU MDR is both a milestone and a stepping stone toward Fincare’s vision of a high-quality global healthcare ecosystem. Moving forward, Fincare will continue to uphold its core philosophy of “Quality as the Foundation, Compliance as the Priority, and Users as the Focus.” We are committed to providing global patients and partners with safer, more reliable, and innovative medical device products—contributing to a healthier, more efficient, and sustainable medical system for all.