Reflecting on a Successful Participation at the 91st CMEF International Medical Equipment Fair
The 91st China International Medical Equipment Fair (CMEF), held from April 8th to 11th, 2025, at the National Exhibition and Convention Center (Shanghai), proved to be an exceptional platform for global medical industry leaders to showcase innovations, exchange insights, and foster business collaborations. As a leading Chinese home medical device manufacturer with 13 years of industry expertise, Finicare Medical proudly participated under Booth 6.1M09, presenting our cutting-edge products and reinforcing our reputation as a trusted OEM/ODM partner and top-rated global seller.

This report summarizes our exhibition experience, customer interactions, product innovations, corporate strengths, and future exhibition strategies, highlighting how we leveraged CMEF to strengthen our brand and expand our international footprint.
Table of Contents:
1. Exhibition Overview & Key Achievements
2. Customer Reception & Market Feedback
3. Product Innovation & Competitive Edge
4. Corporate Strength & Trust Building
5. Future Exhibition Strategy & Goals
1. Exhibition Overview & Key Achievements
CMEF, as the largest medical trade fair in Asia, attracted over 4,000 exhibitors and 120,000 professional visitors from 150+ countries. Our strategically located booth in the 6.1 Hall (International Zone) ensured high visibility among distributors, hospitals, and e-commerce platforms. Key outcomes included:
●500+ targeted client engagements, including bulk buyers from Europe, North America, and emerging markets (e.g., Southeast Asia, Middle East).
●60+ serious inquiries for OEM/ODM partnerships, with 30% advancing to quotation stages.
●15+ potential distributors negotiating exclusivity agreements for regional markets.
●Media coverage from industry journals and live demonstrations for our Amazon #1 bestselling thermometers and blood pressure monitors.



2. Customer Reception & Market Feedback
Our booth’s design emphasized interactive product displays and live demos, allowing visitors to experience:
●Accuracy & Innovation: Clients tested our FDA 510(k)-cleared blood oxygen monitors and CE MDR-certified wrist blood pressure devices, praising their clinical-grade precision.
●E-Commerce Synergy: Amazon-focused buyers valued our verified sales leadership (Top 1 in thermometers/BP monitors) and seamless logistics support.
●Customization Demand: Emerging markets requested multi-language interfaces, bespoke packaging, and FDA/TGA-compliant variants.
Notable Feedback:
European distributors highlighted our MDSAP certification as a key differentiator for regulatory compliance.
Hospital procurement teams showed interest in our Philips-audited production lines for tender eligibility.

3. Product Innovation & Competitive Edge
We spotlighted 6 flagship categories, each addressing global healthcare trends:
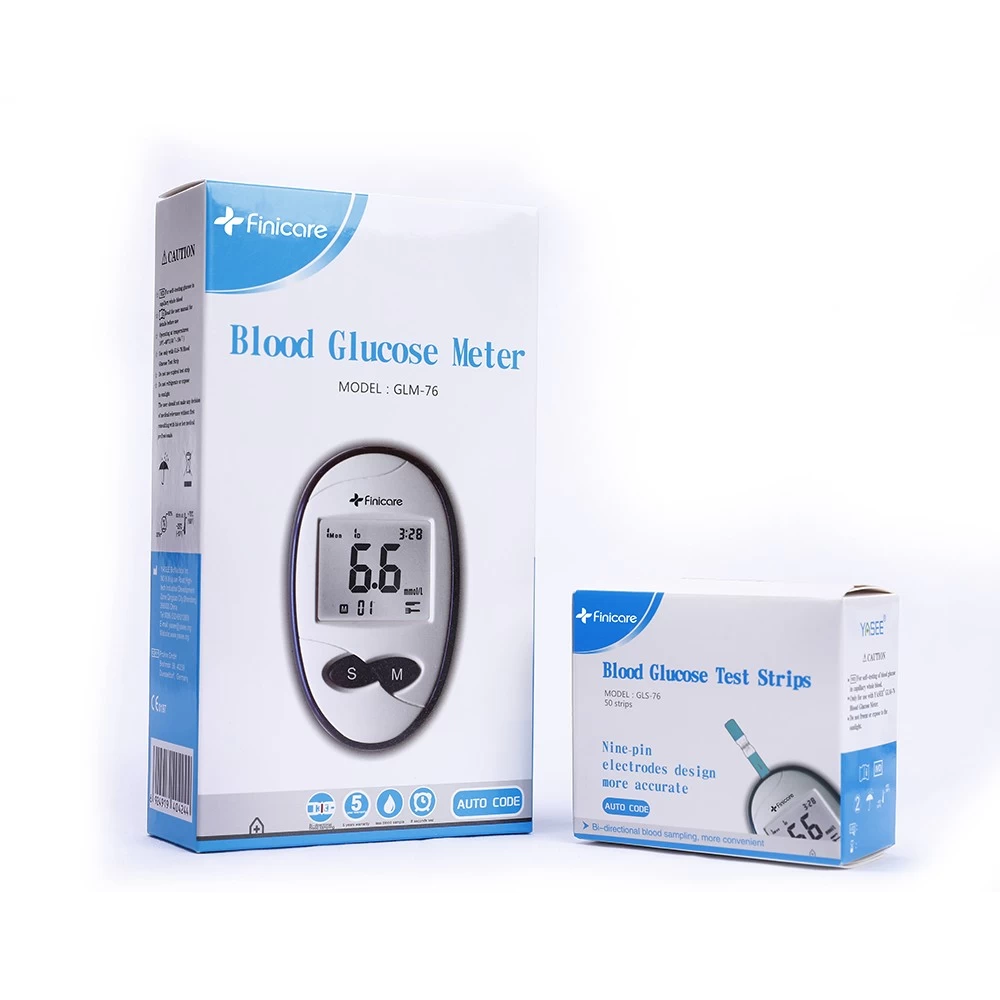
A. Smart Thermometers
●Non-contact Infrared Thermometer: <0.2°C deviation, 1-second reading, pediatric-friendly design.
●Bluetooth-enabled Electronic Thermometer: Syncs with EHR systems, ideal for telemedicine.
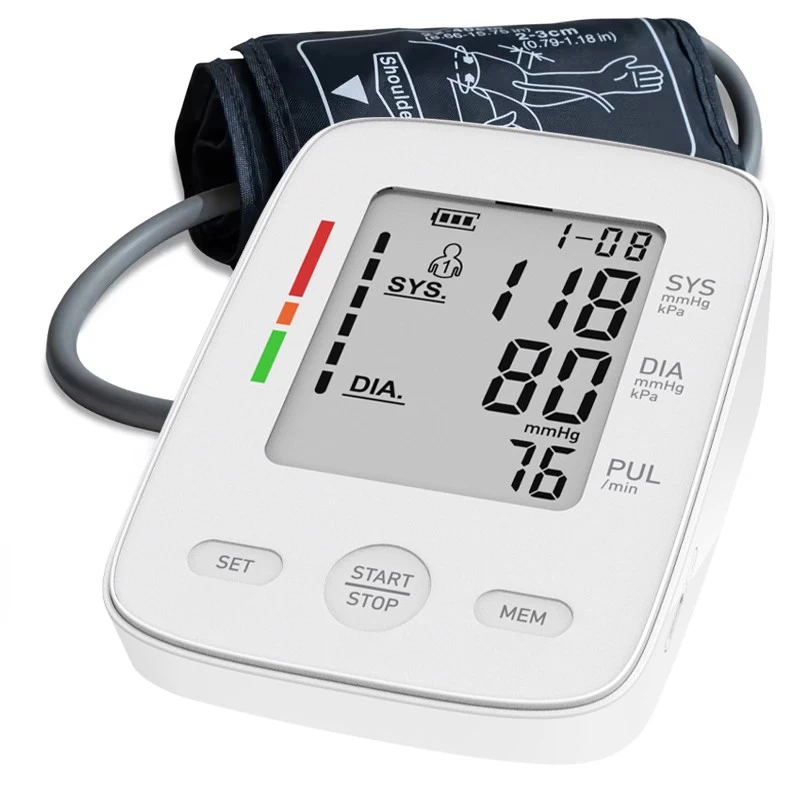
B. Blood Pressure Management
●Arm-type & Wrist BP Monitors: Featuring irregular heartbeat detection and 1000-memory storage (compliant with AAMI/ESH/ISO 81060-2 standards).
C. Pulse Oximeters & Nebulizers
●FDA-listed fingertip oximeters with Perfusion Index (PI) measurement for critical care.
●Ultrasonic mesh nebulizers (30% quieter than competitors, CE MDD compliant).
D. USP Highlights:
●R&D Investment: 8% of annual revenue funneled into IoT/AI integration (e.g., app-connected devices).
●Certifications: ISO 13485, BSCI, TGA, MDL, ensuring cross-market Admission.

4. Corporate Strength & Trust Building
Our branding focused on vertical integration and compliance excellence:
●Manufacturing Prowess: 200,000 sq. ft. facility with SMT/automated assembly lines, serving 2M+ units/year.
●Quality Assurance: MDSAP audited, with 100% pre-shipment inspection.
●Strategic Partnerships: Long-term OEM collaborations with Philips, Walmart, and Top 10 e-commerce brands.
Client Testimonial:
“Your ability to deliver FDA submissions in 90 days—plus Amazon-ready packaging—saves us 6 months of groundwork.” — Medical Director, U.S. Distributor
5. Future Exhibition Strategy & Goals
Building on CMEF’s momentum, we plan to:
●Expand Trade Show Presence: Target MEDICA 2025 (Düsseldorf) and Arab Health 2026 to penetrate EMEA deeper.
●Enhance Digital Engagement: Launch 3D virtual booths for remote client onboarding.
●R&D Roadmap: Develop AI-driven diagnostic devices (e.g., hypertension prediction algorithms).

6.Conclusion
The 91st CMEF solidified our position as a globally compliant, innovation-driven home medical solutions provider. By aligning Finicare Medical certifications, manufacturing scalability, and e-commerce dominance with market needs, we’re poised to capture the $45B home healthcare devices market (CAGR 6.2%, 2025–2030).
Gratitude: We thank our team, clients, and CMEF organizers for this milestone event. Together, we’re advancing accessible, precision healthcare worldwide.