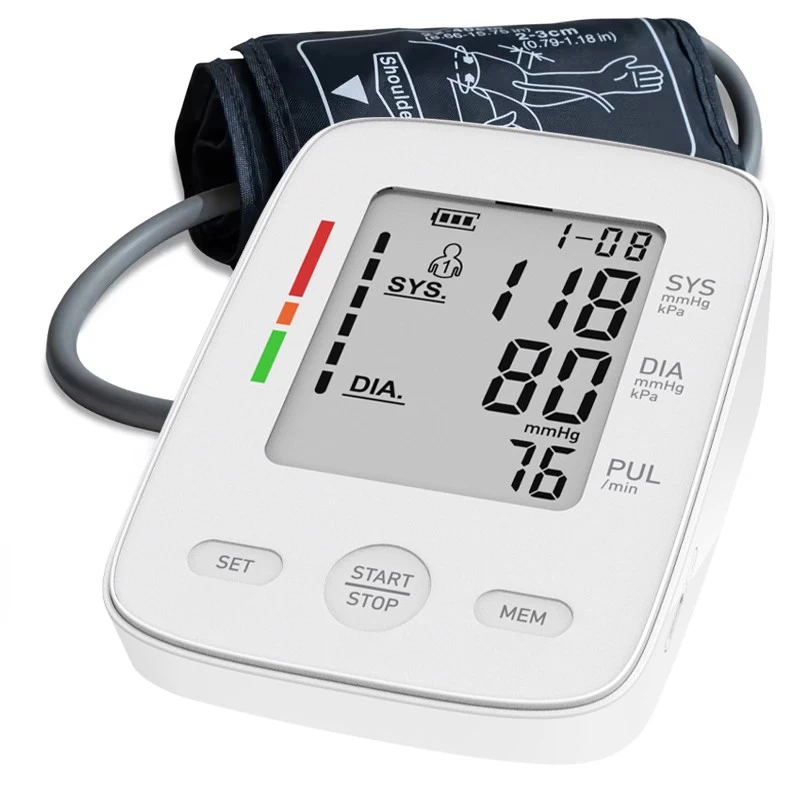
Do FDA-Approved Blood Pressure Monitors?

An FDA-approved blood pressure monitor is a device that has been reviewed and approved by the U.S. Food and Drug Administration. This approval ensures the monitor meets specific safety and accuracy standards. These devices are used to measure blood pressure at home or in a medical setting.
They come in various types, including upper arm, wrist, and finger monitors. The upper arm monitors are generally considered the most accurate. Users can track their blood pressure readings over time, helping manage conditions like hypertension.
FDA approval provides confidence that the device is reliable and effective for personal or clinical use. Always follow the manufacturer’s instructions for best results and consult a healthcare provider for accurate interpretation.
Table of Contents
1.What does FDA approval mean for a blood pressure monitor?
2.Why FDA-Approved Blood Pressure Monitors Better?
3.Does the FDA approve Blood pressure monitors?
4.Are all blood pressure monitors FDA-approved?
5.What is the difference between an FDA-approved and Non FDA-approved Blood pressure FDA monitor?
6.Why is FDA approval necessary for blood pressure monitors?
7.How do I know if a blood pressure monitor is FDA-approved?
8.Conclusion
1.What does FDA approval mean for a blood pressure monitor?
FDA approval for a blood pressure monitor means that the U.S. Food and Drug Administration has thoroughly reviewed the device. This review ensures the monitor meets specific safety, accuracy, and reliability standards. The FDA checks that the device performs as advertised and poses no significant risks to users.
When a blood pressure monitor is FDA-approved, it gives consumers confidence that the readings are accurate and dependable. This is crucial for managing health conditions like hypertension. The approval process involves rigorous testing and evaluation so users can trust that the monitor is effective for its intended use.
FDA approval also means that the manufacturer follows good manufacturing practices. This ensures consistent quality in each device produced. In summary, FDA approval guarantees that a blood pressure monitor is a trustworthy and safe tool for monitoring blood pressure at home or in clinical settings.
2.Why FDA-Approved Blood Pressure Monitors Better?
Here are the reasons why FDA approved Blood pressure monitors are better:
- Ensures precise blood pressure readings, which are crucial for effective health management.
- Meets stringent safety standards, reducing the risk of harm to users.
- It provides consistent and dependable measurements over time.
- Approval from a reputable agency boosts user confidence in the device.
- Manufacturers follow strict quality control practices, ensuring high standards.
- It is often tested and validated in clinical settings for medical use.
- It comes with clear instructions and user-friendly features, enhancing usability.
- It aids in effectively monitoring and managing conditions like hypertension.
- Meets regulatory requirements, ensuring the device’s legality and compliance.
- Typically, it comes with better customer support and warranty options, offering peace of mind.

3.Does the FDA approve Blood pressure monitors?
An FDA-approved blood pressure monitor is a device that has been reviewed and approved by the U.S. Food and Drug Administration. This approval means the monitor meets strict safety and accuracy standards set by the FDA.
These devices are commonly used at home or in medical settings to measure blood pressure. Different blood pressure monitors include upper arm, wrist, and finger monitors. Upper-arm monitors are generally considered the most accurate and reliable.
FDA approval assures that the device has been tested for accuracy and safety. This approval is necessary because it helps users trust the readings they get from the monitor. Regularly using an FDA-approved blood pressure monitor can help individuals track their blood pressure over time, which is crucial for managing conditions like hypertension.
These monitors are easy to use. Most come with clear instructions; some even have digital displays and memory functions to store past readings. However, for the best results, it’s important to follow the manufacturer’s instructions.
4.Are all blood pressure monitors FDA-approved?
Not all blood pressure monitors are FDA-approved. FDA approval indicates that a device has met strict standards for accuracy, safety, and effectiveness. Some monitors may not undergo this rigorous process, and, therefore. lack FDA approval.
These unapproved devices might still be available, but their accuracy and reliability can be uncertain. Consumers need to check for FDA approval when choosing a blood pressure monitor. This ensures they are using a device that has been thoroughly tested and meets high standards.
The Finicare Medial FC-BP121 is an FDA-approved automatic blood pressure monitor used in hospitals. It measures blood pressure using the oscillometric method, which detects vibrations from blood flow in the arteries. This method helps the device give consistent and accurate results in 25-30 seconds.
5.What is the difference between an FDA-approved and Non FDA-approved Blood pressure FDA monitor?
Traditional Vs FDA approved blood pressure monitors

|
FDA Approved BP Monitors |
Non FDA-Approved BP Monitors |
|
Tested and proven to provide accurate readings. |
It may not have undergone rigorous testing for accuracy. |
|
Meets strict safety standards to protect users. |
May not meet stringent safety standards, posing potential risks. |
|
Consistently delivers dependable results over time. |
It can be inconsistent and less dependable in terms of results. |
|
It is manufactured under stringent quality assurance practices. |
May lack consistent quality assurance in manufacturing. |
|
It is often tested and validated in clinical settings. |
Often not tested or validated in clinical settings. |
|
Complies with U.S. regulations and standards. |
Does not comply with FDA regulations and standards. |
|
Higher trust and confidence among users due to FDA approval. |
May offer limited customer support and warranty options. |
|
Typically, it comes with better customer support and warranty options. |
May offer limited customer support and warranty options. |
6.Why is FDA approval necessary for blood pressure monitors?
FDA approval for blood pressure monitors is crucial for several reasons. Firstly, it ensures that the device has undergone rigorous testing to verify its accuracy in measuring blood pressure. This is essential because accurate readings are vital for diagnosing and managing conditions like hypertension, which affects millions worldwide.
It guarantees that the monitor meets strict safety standards, minimizing the risk of user harm. Blood pressure monitors without FDA approval may not have undergone such comprehensive safety evaluations, potentially compromising user safety.
FDA-approved monitors are more likely to provide consistent and reliable results over time. This reliability is essential for monitoring blood pressure trends and making informed decisions about treatment and lifestyle changes.
It signifies that the manufacturer follows good manufacturing practices, ensuring consistent quality in each device. This consistency helps build trust among healthcare professionals and patients alike, knowing they can rely on the monitor’s performance.

7.How do I know if a blood pressure monitor is FDA-approved?
To determine if a blood pressure monitor is FDA-approved, look for specific indications on the device or its packaging. FDA-approved monitors typically display an FDA logo or statement directly on the product. Additionally, manufacturers often mention FDA approval in their product descriptions, manuals, or websites.
You can also verify the device’s status on the FDA’s official website or contact the manufacturer directly for confirmation. Ensuring FDA approval ensures the monitor has met stringent standards for accuracy, safety, and reliability, providing peace of mind when monitoring your blood pressure.
Both the Finicare Medical FC-BP121 and FC-BP113 blood pressure monitors are FDA-approved. It means they meet strict standards for accuracy and safety. It shows their commitment to providing reliable healthcare technology. The Finicare Medical FC-BP121 has been proven in clinical tests and follows ISO standards.
The Finicare Medical FC-BP121 uses oscillometric technology to give precise readings. Customers can trust that both monitors provide consistent, FDA-certified results. Finicare ensures that its products meet high standards for better health monitoring, whether for personal use or in medical settings.
FDA-approved blood pressure monitors are crucial because they ensure accuracy, safety, and reliability. Accurate readings are vital for diagnosing and managing conditions like hypertension. Safety standards protect users from potential harm, and reliability ensures consistent performance over time.
FDA approval means the device has undergone rigorous testing and meets strict quality control practices. This approval gives users and healthcare providers confidence in the device’s performance. Individuals can trust that they use a high-quality tool for effective health management by choosing an FDA-approved monitor.
If you’re looking for the best one, choose the Finicare Medical FC-BP121 and FC-BP113 for reliable, FDA-approved blood pressure monitoring. Both models ensure accuracy, safety, and consistent results. The FC-BP113 is clinically proven and ISO compliant, while the FC-BP121 uses advanced oscillometric technology—Finicare Medical for top-quality health monitoring.